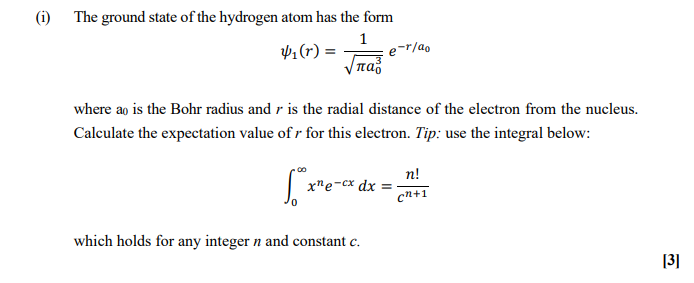
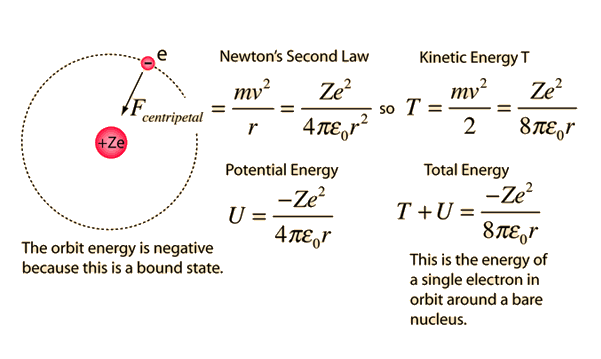
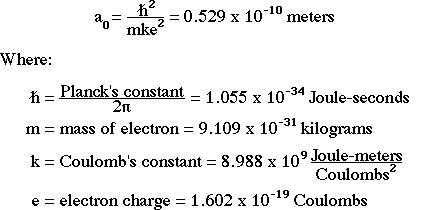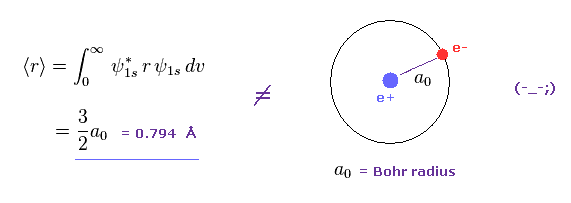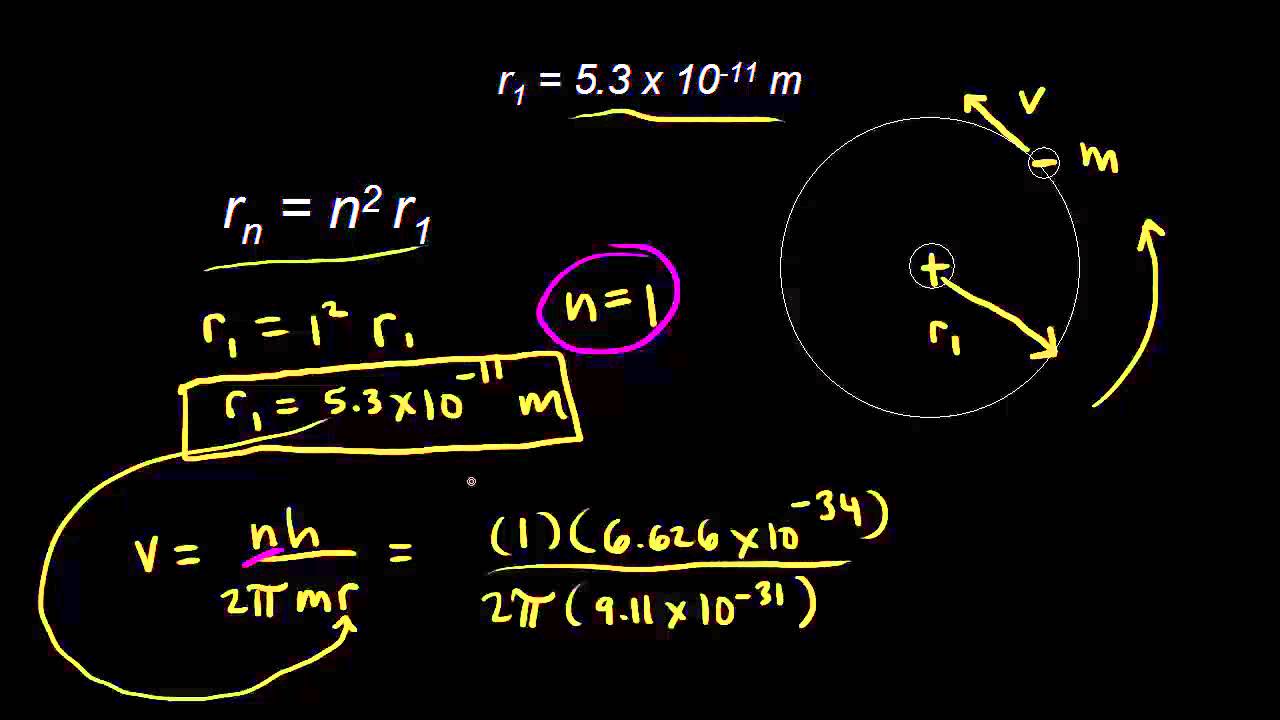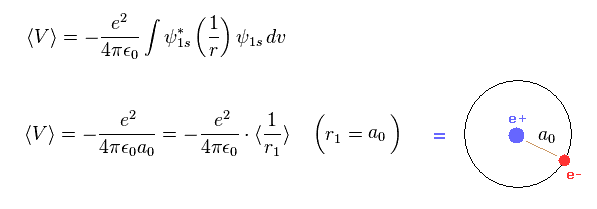Show all steps of the derivation: Show that the most probable radius for an electron described by the function R_10 is the Bohr radius, a_0. | Homework.Study.com

In the ground state of hydrogen atom, its Bohr radius is 5.3xx10^(-11)m. The atom is excited such that the radius becomes 21.2xx10^(-11)m. Find the value of principal quantum number and total energy
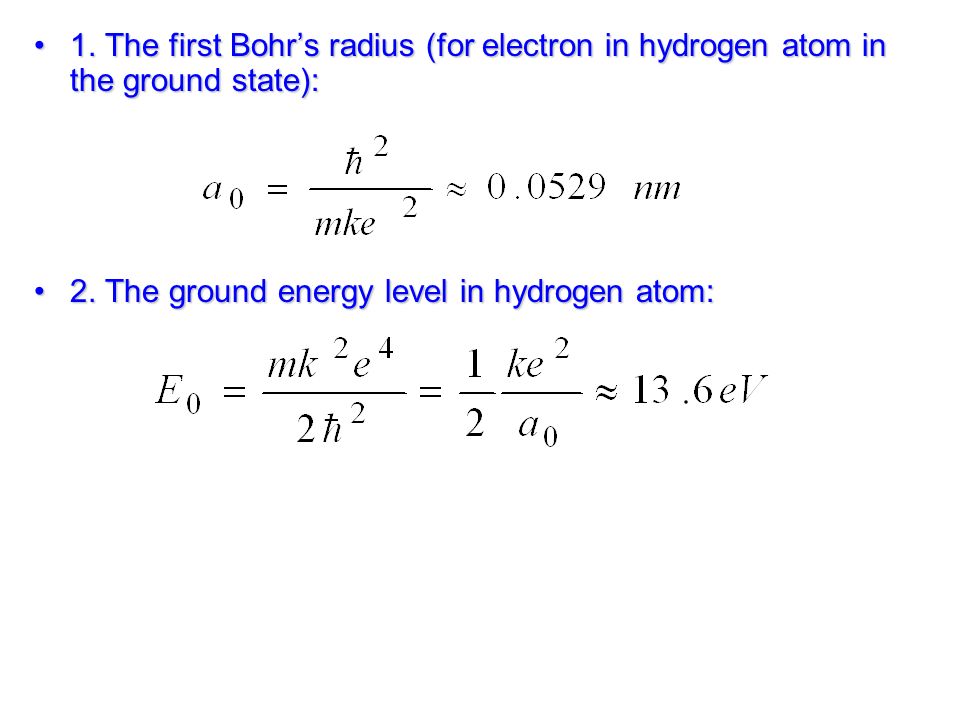
1. The first Bohr's radius (for electron in hydrogen atom in the ground state): 2. The ground energy level in hydrogen atom: - ppt video online download

In the Bohr's model of hydrogen - like atom the force between the nucleus and the electron is modified as F = e^24piε0 ( 1r^2 + betar^3 ) , where beta is

Determining Radius Of An Orbit Using Bohr's Equation | 1st year Chemistry | swap education portal - YouTube

Question Video: Understanding the Effects of Electron Mass on Orbital Radius Using the Bohr Model | Nagwa
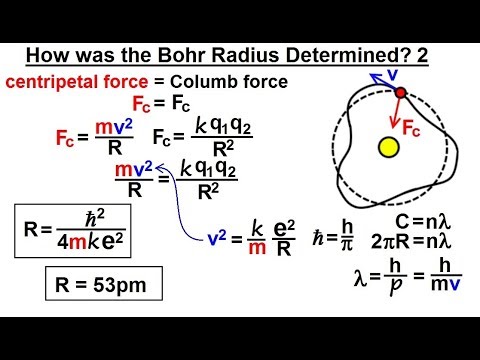
Physics - Ch 66.5 Quantum Mechanics: The Hydrogen Atom (6 of 78) How was the Bohr Radius Determined2 - YouTube